Mpox Virus E5 is a pivotal enzyme that plays a crucial role in the initiation of DNA replication through its helicase activity. This unique viral helicase-primase fusion protein demonstrates an intricate mechanism of DNA unwinding, a process essential for replicating the genetic material of the virus. By employing advanced cryo-EM structures, researchers have illuminated how Mpox Virus E5 orchestrates DNA replication, showcasing its ability to separate the double-stranded DNA (dsDNA) helix and form an active double hexamer. Each step of this DNA uncoiling is driven by ATP cycles, ultimately facilitating the transitioning of dsDNA into single-stranded DNA (ssDNA), which primes the stage for further replication. Understanding the catalytic action of Mpox Virus E5 not only enhances our knowledge of viral replication processes but also suggests potential avenues for therapeutic interventions against viral infections.
The helicase-primase enzyme known as Mpox Virus E5 is an intriguing focus of molecular biology research. This enzyme initiates the crucial task of unwindong DNA helices, which is a foundational step in the complex process of DNA replication. By capturing the cryo-EM structures of E5, scientists have revealed significant insights into its helicase activity and the dynamics of DNA strand separation. The interplay between the helicase and primase functions emphasizes the sophisticated evolutionary adaptations that allow viruses to effectively replicate their genomes. Understanding this enzyme’s role enhances our comprehension of the broader mechanisms of DNA replication, especially in various biological contexts.
Understanding mpox Virus E5 and Its Role in DNA Unwinding
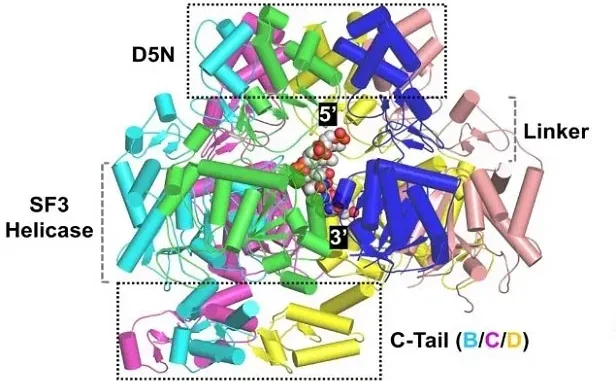
The Mpox Virus E5 protein plays a pivotal role in the initiation of DNA unwinding, a crucial first step in DNA replication. By forming a head-to-head double hexamer around double-stranded DNA (dsDNA), E5 induces conformational changes that facilitate the separation of the DNA strands. This mechanism is vital for creating the single-stranded DNA templates necessary for primase activity and subsequent DNA-dependent polymerase functions. Such understanding opens new avenues in virology, particularly in elucidating how poxviruses replicate within their host cells.
Recent cryo-EM structures reveal the intricate details of how E5 operates. The protein’s unique helicase-primase fusion not only demonstrates traditional helicase activity, characterized by DNA unwinding, but also reveals its dual capability to synthesize RNA primers. This combination of functions exemplifies the evolutionary advantage that such fusions may provide to viral pathogens, ensuring efficient replication under competitive conditions.
The Mechanism of Helicase and Primase Activity in E5
E5’s helicase and primase activities are intricately linked to the precise unwinding of DNA during replication. The helicase domain continuously interacts with the primase domain, enhancing its functionality by providing a scaffold that stabilizes ssDNA. This interaction exemplifies the helicase-primase fusion model found in E5, where the efficiency of enzyme activity is significantly improved. By analyzing ATP cycles within the helicase domain, researchers have identified how nucleotide incorporation drives these structural changes and, in turn, assists in DNA unwinding.
The biochemical characterization of E5 highlights its efficiency and versatility; not only does it perform helicase functions, but it also engages in the synthesis of RNA primers—indicating an evolutionary advantage. As the complex mechanisms of DNA replication continue to be unveiled, insights into E5’s dual role can potentially inform therapeutic strategies against poxvirus infections by targeting these fundamental processes.
Cryo-EM Structures Reveal Insights into E5 Functionality
The use of cryo-electron microscopy (cryo-EM) has revolutionized our understanding of the E5 protein’s functionality. The purified MPXV E5 displayed multiple conformations depending on the binding of DNA and ATP. This structural variability is essential for the helicase activity that unwinds DNA, thus enabling the efficient creation of templates for primase activity. The cryo-EM maps show the distinct states of the enzyme during its catalytic cycle, highlighting the importance of conformational flexibility in its function.
By observing the transitions between different structural forms of E5, researchers can infer the precise dynamics underlying helicase-primase interactions. Such detailed exploration not only sheds light on the molecular mechanisms at play during DNA unwinding but also poses questions about the evolutionary implications of such adaptations in viral environments, where rapid replication is often necessary for survival.
The Evolutionary Importance of Helicase-Primase Fusions in Viruses
The evolutionary preservation of the helicase-primase fusion, particularly in Mpox virus E5, hints at a significant advantage in the viral replication process. These fusions streamline the replication machinery, reducing the need for multiple separate protein factors, which can be a critical factor in the competitive viral landscape. The study of such fusions provides insights into how viruses have adapted their replication strategies to enhance their survival and propagation.
Moreover, understanding the evolutionary background of these enzyme fusions can also foster the development of antiviral strategies. By targeting specific functions of the E5 protein or its structural features that have evolved specifically for replication efficiency, novel therapeutic interventions can be devised to combat infections caused by poxviruses. This cross-disciplinary understanding between evolutionary biology and molecular virology is vital for advancing healthcare.
Helicase Activity in DNA Replication: Focus on E5
Helicase activity is fundamentally crucial for the initiation of DNA replication, where the unwinding of dsDNA occurs. Mpox virus E5 exemplifies this as it demonstrates both helicase and primase functions, making it a unique model for studying replication processes. Understanding the mechanisms of E5’s helicase activity helps researchers decipher the initial steps of DNA unwinding, which are paramount for subsequent replication and cell division.
In the context of Mpox virus E5, the DNA unwinding process is complemented by helicase-primase fusion, enhancing the ability to rapidly generate necessary components for replication. The study of helicase activity and its regulation within E5 adds valuable insight to the broader understanding of DNA replication across various organisms, expressing the importance of such mechanisms in viral biology.
Implications of DNA Unwinding on Viral Evolution
The process of DNA unwinding not only facilitates replication but also influences the evolutionary trajectory of viruses. The Mpox virus E5 protein’s ability to efficiently unwind DNA and synthesize RNA primers means that it can rapidly adapt to host environments and replicate swiftly. This ability showcases the relationship between the DNA replication mechanisms of viruses and their evolutionary success, creating competitive advantages in various ecological niches.
Further investigation into E5-mediated DNA unwinding processes might reveal additional evolutionary pressures applied to viral replication strategies. Understanding these dynamics could inform the development of novel antiviral therapies that disrupt the replication processes of poxviruses and related pathogens, ultimately contributing to global health outcomes.
Biochemical Studies of E5: Insights and Discoveries
Biochemical assays are essential in characterizing the multifunctionality of the Mpox virus E5 protein. Such studies have shown that E5 not only exhibits helicase activity but can also catalyze RNA primer synthesis, suggesting a dual role within the viral replication cycle. The findings highlight the versatility of viral enzymes and their adaptive functions, crucial for maintaining the efficiency of DNA replication in varying environments.
Furthermore, the identification of E5 as a member of the PrimPol family—known for dual RNA and DNA synthesis roles—underlines the evolutionary significance of enzyme specialization in viruses. This understanding encourages further research into the biochemical pathways utilized by other viral helicases, contributing to a more comprehensive view of viral replication strategies and potential therapeutic targets.
Research Methods for Characterizing E5 Function
Characterizing the function of the Mpox virus E5 protein involved meticulous protein expression and purification techniques, utilizing the Bac-to-Bac baculovirus expression system. Such methodologies are critical for obtaining high-quality protein samples necessary for functional assays to delineate its helicase and primase properties. Understanding the specific biochemical behaviors of E5 within the context of DNA unwinding informs future studies and applications in virology.
Additionally, structural analysis through cryo-EM provides a powerful means to visualize the dynamics of the E5 protein during various enzymatic conditions. This combination of biochemical characterization and advanced microscopy techniques allows researchers to comprehensively evaluate the mechanisms by which E5 operates in DNA replication, leading to discoveries that have profound implications for our understanding of viral biology and evolution.
Future Directions in mpox Virus Research
As research continues on the Mpox virus E5 protein and its helicase-primase functionality, future studies should aim to explore the specific molecular interactions that govern its activity. Investigating the detailed mechanisms of E5’s dual roles in DNA unwinding and RNA primer synthesis could lead to breakthroughs in understanding viral replication and the potential for developing targeted antiviral therapies.
Additionally, expanding upon the evolutionary implications of these enzyme fusions across different viral families might elucidate broader themes in viral adaptations. By integrating evolutionary biology with molecular virology insights, researchers can uncover novel strategies for combating viral infections, ultimately advancing our capabilities in public health and disease management.
Frequently Asked Questions
What is the role of the Mpox Virus E5 helicase in DNA unwinding?
The Mpox Virus E5 helicase plays a crucial role in DNA unwinding by initiating the separation of double-stranded DNA (dsDNA) during replication. Its helicase activity is vital for the recruitment of primase and polymerase, facilitating the formation of the replisome needed for DNA replication.
How does the helicase-primase fusion of Mpox Virus E5 function in DNA replication?
The helicase-primase fusion of Mpox Virus E5 enhances its functionality, enabling it to perform dual roles: unwinding dsDNA and synthesizing RNA primers. This unique fusion promotes efficient DNA replication by coordinating helicase activity with primase action, ensuring timely primer synthesis.
What are the intricate structures observed in the Mpox Virus E5 during DNA unwinding?
Cryo-EM structures of Mpox Virus E5 reveal a head-to-head double hexamer configuration that encircles dsDNA. These structures illustrate the conformational changes that occur during DNA unwinding, pivotal for understanding the helicase’s mechanism in DNA replication.
How does ATP influence the helicase activity of Mpox Virus E5?
ATP binding induces conformational changes in Mpox Virus E5, driving its helicase activity during DNA unwinding. The hydrolysis of ATP is critical for facilitating the translocation of the helicase along ssDNA, which is integral to the DNA replication process.
What are the evolutionary implications of the helicase-primase fusion in Mpox Virus E5?
The functional association of helicase and primase domains in Mpox Virus E5 offers insights into evolutionary preservation strategies among viruses. This fusion model provides a simplified version of the complex interaction seen in cellular systems, highlighting adaptations that enhance viral replication efficacy.
Can the mechanisms of Mpox Virus E5 be applied to understanding other dsDNA viruses?
Yes, the insights gained from studying Mpox Virus E5’s helicase-mediated DNA unwinding can inform our understanding of other dsDNA viruses. Their replication mechanisms may share similarities, making E5 a model for investigating helicase-primase interactions across various viral species.
What experimental methods were used to characterize the Mpox Virus E5 helicase activity?
The characterization of Mpox Virus E5 helicase activity involved biochemical assays, protein expression and purification using the Bac-to-Bac baculovirus system, and structural analysis through cryo-EM to elucidate the enzyme’s functionality during DNA replication.
| Key Points |
|---|
| Research on Mpox Virus E5 explores its role as a helicase-primase that catalyzes DNA unwinding initiation. |
| E5 forms a head-to-head double hexamer, crucial for DNA unwinding and primer synthesis. |
| Study utilizes cryo-EM structures to reveal E5’s enzymatic mechanisms and transitions during DNA replication. |
| E5 exhibits multifunctional abilities, including helicase and DNA-dependent polymerase activities using NTPs and dNTPs. |
| Findings provide insights into evolutionary preservation of helicase-primase fusions and their role in dsDNA virus replication. |
Summary
The Mpox Virus E5 is instrumental in the initiation of DNA unwinding during replication, exhibiting unique helicase-primase functionalities that reflect evolutionary adaptations in viral biology. This comprehensive study of Mpox Virus E5 elucidates the complex mechanisms that govern DNA replication, highlighting its head-to-head double hexamer structure and multifunctionality. Understanding these processes paves the way for future investigations into antiviral strategies and the evolutionary dynamics of helicase-primase fusion proteins.
Leave a Reply